The pharmaceutical industry is currently undergoing a digital transformation to improve its supply chain planning capabilities, leveraging new technologies. These efforts are crucial for safeguarding patient well-being amidst constantly evolving regulatory demands and supply chain disruptions. Drawing from client interactions and our understanding of ongoing supply chain challenges, we have outlined the top ten requirements that companies must address for a successful transformation. Neglecting these aspects poses a risk to the digital roadmap, potentially resulting in unsuccessful implementation and missed business benefits.

Figure 1 represents the top 10 planning requirements from CAMELOT’s perspective. These requirements are not listed in order of importance. Importance varies by company depending on the complexity of their supply chain and scope of implementation. However, all ten are applicable in any case.
- Artwork Constraints: Consider artwork approval throughout planning to optimize the packaging plan and determine the most suitable packing dates (with the new artwork), all in alignment with market requirements and current inventory levels.
- Tender Management: Assess the feasibility of participating in one or multiple new tenders through understanding the current network capabilities (inventory and available capacity).
- Sequencing and Resource Optimization: Find the optimal production sequence considering product allocation (in case of alternate resources) as well as resource and campaigning constraints.
- Quality Assurance/ Quality Control (QA/QC) Planning: Consider inspection lead times and lab capacity constraints for QA/QC processes in planning.
- CMO Integration: Integrate the most relevant information related to CMO operations into the current plan (delivery dates, inventory, etc.).
- Inventory Visibility and Optimization: Define buffer levels and decoupling points to optimize inventory across their network considering lead times, shelf-life, regulatory constraints, and global and local policy stock strategies.
- Clinical Planning: Model and plan clinical processes in conjunction with commercial production to identify and prioritize activities according to business strategy.
- Supply Chain Risk Management: Understand the current risk level within the supply chain and identify its vulnerabilities in order to proactively configure the network to cope with the anticipated level of risk.
- Life Cycle Management: Identify the product life cycle status and define different planning approaches for supply and demand considering market approval and validation dates, launch and phase-out strategies, and seasonality.
- Regulatory Requirements: Consider regulatory constraints, such as regulatory approval dates and product restrictions, across the network and understand the end-to-end impact of a regulatory change.
Impact on the Pharmaceutical Supply Chain
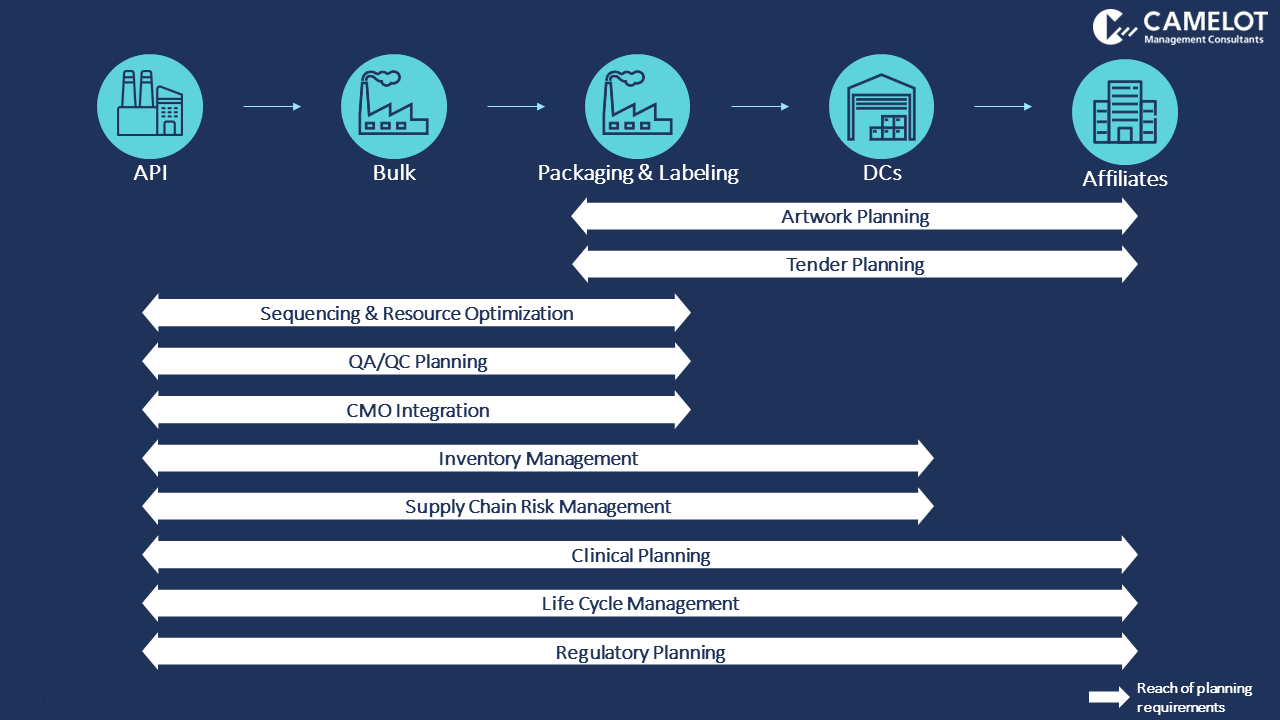
Figure 2 maps the impact each requirement has on the supply chain. This portrays the parts of the supply chain that need to be involved, or at least considered, when defining solutions to approach these requirements. The indicated scope of the supply chain must undergo a complete transformation for the business to meet all expected benefits. The extent of the reach for each requirement will vary between companies, depending on factors like their product mix and network complexity.
Conclusion
With these selected requirements, our goal is not to diminish the importance of the general planning requirements, such as automated forecasting and constrained supply planning, which are vital for implementing a comprehensive end-to-end supply chain planning process. Instead, we aim for this selection to be viewed as essential components that planners should integrate when designing and implementing the end-to-end planning processes and digital solution landscape in the pharmaceutical industry. This will ensure that the digital transformation returns the expected business benefits.

